I do a lot of consulting work for food entrepreneurs and small food companies. One thing I’ve noticed most of these people miss is just how important understanding pH and acidity is.
Not only does the pH of a food often determine its shelf life (since it influences microbial growth), it can also impact the taste and texture.
In this post, I’ll break down the science of pH, its role in cooking, and why it’s crucial for food preservation. We’ll also look at how pH and acidity affect flavors, baking, and even meat tenderization.
💡 Want to learn more about food science basics? My Food Science for Beginners course covers key concepts like moisture content, water activity, and food preservation. Check it out here.
What is pH?
pH stands for the negative logarithm of the hydrogen ion concentration (H+). The pH scale ranges from 0 (highly acidic) to 14 (highly alkaline), with 7 being neutral.
- Acidic Foods: Almost all food is acidic
- Neutral Foods: Water (pH 7)
- Basic Foods: Baking soda and egg whites (pH ~9)
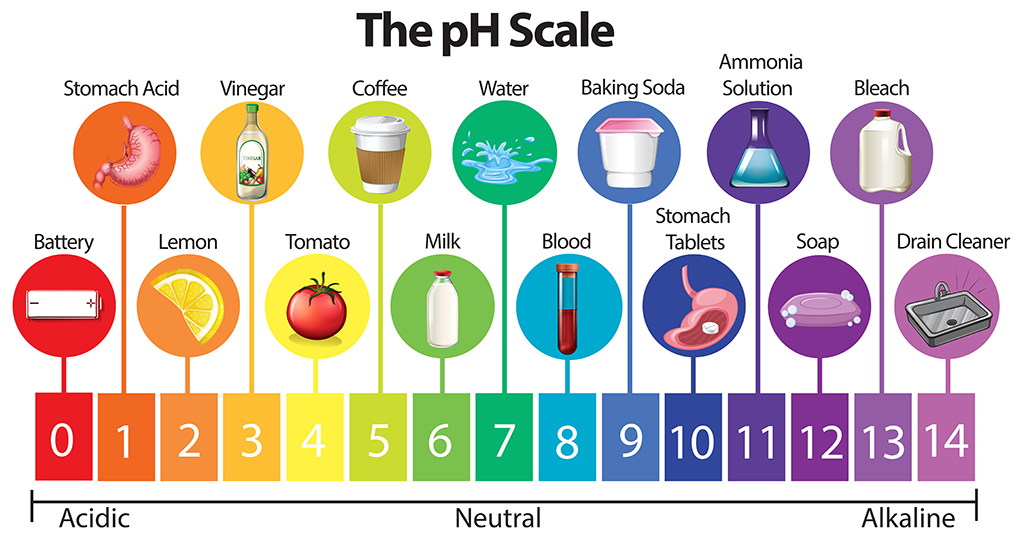

The scale is logarithmic, meaning a difference of one pH unit represents a 10-fold difference in acidity or alkalinity. So, if your food product goes from normally being a pH=6 down to a pH=5, this is a HUGE change!
I would say it’s normal to see small changes from 6.0 to 6.1 and 5.9, but a jump of one whole pH unit means something has been drastically altered.
Food Preservation: The Role of Acidity
I would argue the reason knowing the pH of your food product is that it dictates whether or not microorganisms can grow thus dictating your shelf life.


For example, fermented and pickled foods, such as sauerkraut and yogurt, have low pH levels, which prevent the growth of harmful bacteria. The low pH prevents these foods from spoiling for a very long time.
Funny enough, it is actually lactic acid bacteria that make the lactic acid and ultimately lower the pH of the fermented good, but that’s life for you.
Similarly, controlling pH in canned foods helps avoid microbial contamination and a long shelf life.
Interestingly, the acidity of a product like pickles or sauerkraut not only helps preserve the food but also influences its taste.
Acidity and Taste
Acidity plays a huge role in the taste of food.


When acidic molecules reach our tongue, they trigger our sour taste buds. This is why foods with higher acidity levels, such as citrus fruits and vinegar, tend to be tangy or sour.
I remember eating the candy WarHeads as a child, and just loving the absolute rush of just how sour it was. These candies were simply coated with citric acid powder that delivered a punch when eaten.
When formulating foods, there’s several edible acids we tend to use to lower pH including:
- Citric Acid: Found in citrus fruits like lemons and oranges.
- Acetic Acid: Found in vinegar, pickles, and fermented foods.
- Lactic Acid: Found in dairy products like yogurt and sour cream.
pH affects baking chemistry, especially in leavening. Baking soda (a base) requires an acid to produce carbon dioxide gas, which helps cakes and cookies rise. Common acidic ingredients include buttermilk, vinegar, or lemon juice. Without this reaction, baked goods would lack their light, airy texture.
Similarly, pH plays a role in meat marinades. Acids like vinegar or citrus juice break down proteins, making meat more tender. However, too much acidity for too long can make the meat mushy, so timing is key.
Acidity and Texture
Acidic ingredients also play a key role in gas production and the rise of baked goods. If you are even an amateur baking I’m sure you’ve witnessed this yourself.


Many baked goods from cakes to cookies require a leavening agent like baking soda.
Learn more: Baking soda vs. baking powder
Chemically, baking soda is a base called sodium bicarbonate. When added to a batter, the base needs an acid to produce carbon dioxide bubbles and aerate the food.
Common acidic ingredients include buttermilk, vinegar, or lemon juice. Without this reaction, baked goods would lack their light, airy texture.
Key Takeaways
pH and acidity are at the heart of many food products from pickles to candies!
Understanding pH is more than just a theoretical concept—it’s practical knowledge you can apply in the kitchen.

