Why Does Bread Stale?
And how to reverse it


The disappointment of devouring a fresh baguette for dinner, dreaming about it all night, only to wake up the next day to a stale, rigid loaf is all too familiar. As a bread lover and general lover of all carbohydrates, I feel your pain.
While most of us easily recognize the signs of staling, like a crumbly texture and leathery crust, I’m guessing many don’t have any idea what’s happening inside the bread to create these changes. But, if we can uncover the cause of staling, it’ll be much easier to understand how to reverse it.
And it’s not a simple issue of water loss. It’s really a starch problem.
Starch Chemistry 101
Many of us understand that bread contains something called starch, but we might not be exactly sure what it is or how it functions in baked goods. Well, here’s a little bit of background so we can get to the bottom of this staling issue.
Plants store energy in the form of starch molecules. Starch is produced by connecting hundreds of glucose (sugar) units together to form a larger compound. Most often, starch is concentrated into a granule. Most cereals like wheat, corn, and rice are excellent sources of starch.
Looking a bit more in detail, starch granules are composed of two distinct carbohydrates: amylose and amylopectin. Although both components are made up of glucose units, their structures are quite different.
Amylose is known for its straight chain structure. Each hexagon seen below is a glucose unit. Now, imagine thousands of these glucose molecules linked together to make amylose. The long, linear amylose molecules easily align giving the dough its elasticity and stretchiness.
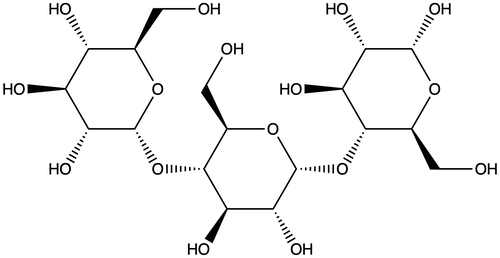

In contrast, amylopectin is a highly branched molecule. It has one long backbone, but imagine branches of all different lengths growing off of the main chain. Amylopectin is big and bulky. It can’t align as nicely as straight-chained amylose.
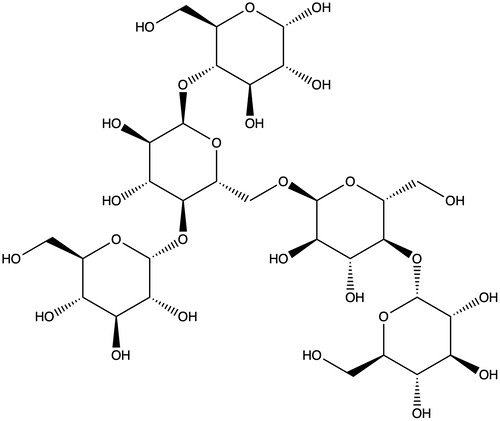

And it’s these structural differences between amylose and amylopectin that make the two carbohydrates act quite differently during baking.
Heat + Water = Starch Gelation
If you’ve ever made homemade bread, you know that the dough you put in the oven looks pretty different than the loaf you have at the end of baking. That’s because during baking, heat or energy is applied to the dough, and this results in chemical changes.
As the dough gets hotter and hotter, molecules begin to vibrate and move, this includes the components of the starch granule. The increased energy causes bonds to break between molecules, allowing them to bounce around more freely.
With the starch granule buzzing with energy, water from the dough can seep into any open voids. As more water enters the granule it begins to swell. This produces perfect conditions for amylose, that linear carbohydrate, to leach out of the starch granule.
This step, the amylose escaping from the starch granule, is absolutely key to the starch gelation seen in baked goods. As more amylose molecules escape, they begin to interact and connect with one another. Eventually, they form a strong, interlocking gel. This is what we call starch gelation.
What’s meant by gelation is that the amylose network is so well connected that it encompasses other components of the bread, like the granules or air bubbles. And once cooled, this amylose network is firm, strong, and helps hold the shape of the loaf.
Staling Begins Now!


It might be disappointing to hear, but as soon as you take that fresh bread out of the oven, the process of staling has already begun. As the bread starts to cool and its temperature drops, the amylose molecules that once gelled to form a beautiful, voluminous loaf of bread, begin to be a detriment.
With time, amylose prefers to associate with other amylose molecules instead of any water. Later, the same thing happens with amylopectin. This is termed starch retrogradation, the more scientific term for staling, and it happens in all starch-based foods.
Once out of the oven, the amylose molecules in the gel network will rearrange themselves until they become closer and closer. The molecules pack tightly together to form a dense aggregate, which eventually crystallizes. It’s these amylose crystals that give stale bread a gritty texture.
As the amylose prefers to interact with other amylose molecules, they break off any interactions they previously had with water. Once the water has been freed, it can easily leave the bread entirely, resulting in a dry product.
In the second stage of staling, amylopectin starts to retrograde and also forms crystals.
Together, this results in more crystalline or solid material in the food, as well as a loss of water, explaining the rigid crumb and crunchy texture of stale food.
How to Reverse Staling
The trick to making your stale foods taste fresh again is melting those pesky crystals.
This can be easily done by heating the bread back up. Throw the loaf or a couple slices back in the oven until they reach about 122–140°F. This will allow amylose and amylopectin to move freely again. Of course, once the loaf cools, the crystalline areas will reform, but the reheating method can be used multiple times.
How Commercial, Pre-Sliced Breads Stay Fresh?


Next time you buy packaged bread from the grocery store, take a look at the ingredient statement. It’s a lot longer than any recipe you would use for homemade bread.
The reason being, these loaves of bread must stay fresh for weeks. The bread has to be mass produced, packaged, transported to a grocery store, and then waits on the shelf to be purchased. This isn’t a quick process.
From the ingredient list, you’ll notice most of these commercial breads contain a type of additive called an emulsifier. Typically emulsifiers in bread include sodium stearoyl lactate, monoglycerides, DATEM, lecithin, and calcium stearoyl lactate.
The funny thing is, just how emulsifiers slow staling is still somewhat undetermined. It’s generally thought that the emulsifiers have some direct interaction with the starch, which inhibits retrogradation.
For example, if you see DATEM on the ingredient statement, it’s known for interacting with both amylose and amylopectin to prevent them from coming together and forming crystals. Another emulsifier called lecithin, which is typically extracted from soybeans, is known for its ability to slow down amylopectin crystallization and therefore staling.
By adding some type of emulsifier to bread formulation, the resulting loaf has a shelf life of many weeks compared to only a couple days.
From one bread lover to another, I hope this short trip into the science of staling comes in handy. Don’t forget the little trick of heating stale bread up to make it appear fresh once again. Now go spread that butter, or jam if that’s more your style. Enjoy!
